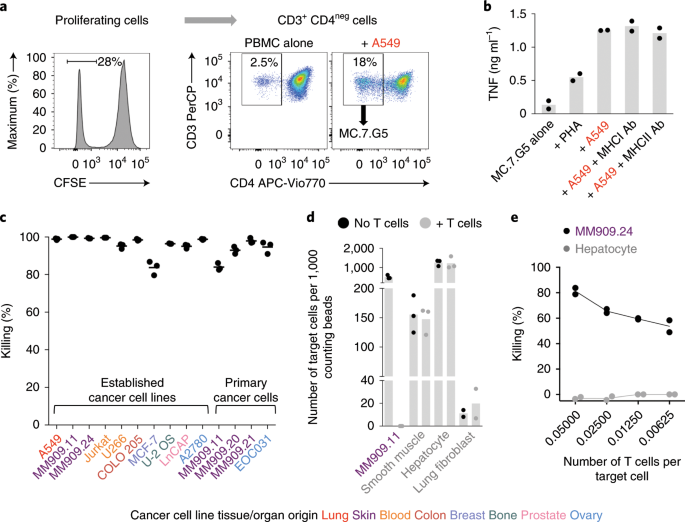
Genome-wide CRISPR–Cas9 screening reveals ubiquitous T cell cancer targeting via the monomorphic MHC class I-related protein MR1
Human leukocyte antigen (HLA)-independent, T cell–mediated targeting of cancer cells would allow immune destruction of malignancies in all individuals. Here, we use genome-wide CRISPR–Cas9 screening to establish that a T cell receptor (TCR) recognized and killed most human cancer types via the monomorphic MHC class I-related protein, MR1, while remaining inert to noncancerous cells. Unlike mucosal-associated invariant T cells, recognition of target cells by the TCR was independent of bacterial loading. Furthermore, concentration-dependent addition of vitamin B-related metabolite ligands of MR1 reduced TCR recognition of cancer cells, suggesting that recognition occurred via sensing of the cancer metabolome. An MR1-restricted T cell clone mediated in vivo regression of leukemia and conferred enhanced survival of NSG mice. TCR transfer to T cells of patients enabled killing of autologous and nonautologous melanoma. These findings offer opportunities for HLA-independent, pan-cancer, pan-population immunotherapies.
What have they found?
Our immune system is our body's natural defence against infection, but it also attacks cancerous cells.
The scientists were looking for "unconventional" and previously undiscovered ways the immune system naturally attacks tumours.
What they found was a T-cell inside people's blood. This is an immune cell that can scan the body to assess whether there is a threat that needs to be eliminated.
The difference is this one could attack a wide range of cancers.
 www.nature.com
www.nature.com
Human leukocyte antigen (HLA)-independent, T cell–mediated targeting of cancer cells would allow immune destruction of malignancies in all individuals. Here, we use genome-wide CRISPR–Cas9 screening to establish that a T cell receptor (TCR) recognized and killed most human cancer types via the monomorphic MHC class I-related protein, MR1, while remaining inert to noncancerous cells. Unlike mucosal-associated invariant T cells, recognition of target cells by the TCR was independent of bacterial loading. Furthermore, concentration-dependent addition of vitamin B-related metabolite ligands of MR1 reduced TCR recognition of cancer cells, suggesting that recognition occurred via sensing of the cancer metabolome. An MR1-restricted T cell clone mediated in vivo regression of leukemia and conferred enhanced survival of NSG mice. TCR transfer to T cells of patients enabled killing of autologous and nonautologous melanoma. These findings offer opportunities for HLA-independent, pan-cancer, pan-population immunotherapies.
What have they found?
Our immune system is our body's natural defence against infection, but it also attacks cancerous cells.
The scientists were looking for "unconventional" and previously undiscovered ways the immune system naturally attacks tumours.
What they found was a T-cell inside people's blood. This is an immune cell that can scan the body to assess whether there is a threat that needs to be eliminated.
The difference is this one could attack a wide range of cancers.

Genome-wide CRISPR–Cas9 screening reveals ubiquitous T cell cancer targeting via the monomorphic MHC class I-related protein MR1 - Nature Immunology
Identifying selective tumor-associated molecules that can act as targets for T cells is a major goal of immunotherapy. Sewell and colleagues demonstrate that the nonclassical MHC molecule MR1 is expressed on a wide variety of cancer types and can be targeted by conventional T cells.