You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Horizons Is it possible for a planet to have liquid water but no atmosphere?
- Thread starter masCh
- Start date
This is just me talking off the cuff using whatever bits and pieces of scientific knowledge I've gathered over the years without citing any references, but wouldn't having liquid water on the surface almost virtually guarantee having an atmosphere? Say an icy comet impacts the surface and introduces liquid water, eventually that water evaporates into water vapor and whether it stays as the beginnings of an atmosphere depends on whether or not the planet has a magnetic field or not. For instance, I thought I read somewhere that the reason Mars doesn't have as much water as we theorize it should have is because it evaporated into the atmosphere, then the atmosphere got gradually stripped away by the solar wind because Mars doesn't have a strong enough magnetosphere to protect it.
I am curious to the reasons why or why not.
Can anyone volunteer a scientific reasoning?
The atmosphere is what protects the planet, it's why you only see rocky airless planets in game. If a planet was to lose its atmosphere then solar radiation, asteroids etc would make short work of anything that didn't evaporate away do to lack of pressure.
I am curious to the reasons why or why not.
Can anyone volunteer a scientific reasoning?
Sure, just look inside our own solar system. Europa has liquid water covered by a thick layer of ice however, its gravity is so low that any gas that leaks out from under the ice floats away into space so, no atmosphere. The only reason there is any liquid water at all on Europa is because of tectonic activity deep inside the planet. And before anyone cries "MOON" it is only a moon because it orbits Jupiter. Europa is larger than Pluto. o7
This is just me talking off the cuff using whatever bits and pieces of scientific knowledge I've gathered over the years without citing any references, but wouldn't having liquid water on the surface almost virtually guarantee having an atmosphere? Say an icy comet impacts the surface and introduces liquid water, eventually that water evaporates into water vapor and whether it stays as the beginnings of an atmosphere depends on whether or not the planet has a magnetic field or not. For instance, I thought I read somewhere that the reason Mars doesn't have as much water as we theorize it should have is because it evaporated into the atmosphere, then the atmosphere got gradually stripped away by the solar wind because Mars doesn't have a strong enough magnetosphere to protect it.
With respect, the OP did not specify water "on the surface". See my post above. o7
Last edited:
On earth, a vapor always forms above the surface of water because molecules of water are always trying to escape and get "pulled back in" to the main body of water.
On another planet without an atmosphere, that vapor would form its own atmosphere, so the planet could not exist without an atmosphere if it had a very large (planetary scale) body of water present. It could, of course, disappear over time as the water evaporates and the atmosphere vents into space, as is suspected in the case of Mars.
Except in the case that JetsonRing has cited, where the water is contained beneath a shell that prevents it forming the vapor.
On another planet without an atmosphere, that vapor would form its own atmosphere, so the planet could not exist without an atmosphere if it had a very large (planetary scale) body of water present. It could, of course, disappear over time as the water evaporates and the atmosphere vents into space, as is suspected in the case of Mars.
Except in the case that JetsonRing has cited, where the water is contained beneath a shell that prevents it forming the vapor.
Last edited:
Water can exist in its frozen state where there's is little or no atmosphere, it can be trapped within a planet from what I have observed/read.
Liquid water is another matter altogether. As nature seeks a balance, surface liquid water w/o the protection of an atmosphere would evaporate and drift away over time. There are scientific explanations on the web.
Liquid water is another matter altogether. As nature seeks a balance, surface liquid water w/o the protection of an atmosphere would evaporate and drift away over time. There are scientific explanations on the web.
The phase diagram of water can probably help here: http://www1.lsbu.ac.uk/water/water_phase_diagram.html (about quarter of the way down the page)
H[SUB]2[/SUB]O doesn't exist as a liquid below about 6 millibars of pressure so if the surface is a complete vacuum then no, there can't be liquid water on the surface.
However if the temperature was high enough the water would sublime into steam rather than forming ice, this might raise the pressure at the surface enough for liquid water to exist (6 millibars really isn't a lot of atmosphere, it's roughly the atmospheric pressure of present-day Mars)
H[SUB]2[/SUB]O doesn't exist as a liquid below about 6 millibars of pressure so if the surface is a complete vacuum then no, there can't be liquid water on the surface.
However if the temperature was high enough the water would sublime into steam rather than forming ice, this might raise the pressure at the surface enough for liquid water to exist (6 millibars really isn't a lot of atmosphere, it's roughly the atmospheric pressure of present-day Mars)
Sure, just look inside our own solar system. Europa has liquid water covered by a thick layer of ice however, its gravity is so low that any gas that leaks out from under the ice floats away into space so, no atmosphere. The only reason there is any liquid water at all on Europa is because of tectonic activity deep inside the planet. And before anyone cries "MOON" it is only a moon because it orbits Jupiter. Europa is larger than Pluto. o7
With respect, the OP did not specify water "on the surface". See my post above. o7
Oh yeah, I forgot about Europa. I even read 2061 too. Long day.
Thanks for the replies & discussion!
I appreciate it now yes water cannot exist in liquid form when the pressure is below the saturation pressure for H2O.
And without an atmosphere pressing down against the surface of the planet, the pressure at the surface will remain near-vacuum.
However, suppose water does form vapour molecules at the surface of the planet. Why can't the planet hold on to these vapour molecules via its gravity?
How does a planet hold on to the nitrogen gases, the methane and CO2 (that results in becoming an atmosphere) but not to H2O vapour?
Suppose we ignore solar winds : a planet without a master star, or a planet very far away from its master star?
I appreciate it now yes water cannot exist in liquid form when the pressure is below the saturation pressure for H2O.
And without an atmosphere pressing down against the surface of the planet, the pressure at the surface will remain near-vacuum.
However, suppose water does form vapour molecules at the surface of the planet. Why can't the planet hold on to these vapour molecules via its gravity?
How does a planet hold on to the nitrogen gases, the methane and CO2 (that results in becoming an atmosphere) but not to H2O vapour?
Suppose we ignore solar winds : a planet without a master star, or a planet very far away from its master star?
However, suppose water does form vapour molecules at the surface of the planet. Why can't the planet hold on to these vapour molecules via its gravity?
I think you're probably describing .. an atmosphere.
However, worlds without atmosphere in ED, do exhibit mists (partial atmosphere) in craters, at certain types of day. IIRC these are volatiles, that escape the rocks when the temp is high enough above (sic. absolute) zero.
I think the argument still holds though, a mist wouldn't provide enough pressure, to prevent any volatiles from subliming ie. the volatile transitions directly from soild form into a gaseous one, missing out the liquid phase altogether.
Thanks for the replies & discussion!
I appreciate it now yes water cannot exist in liquid form when the pressure is below the saturation pressure for H2O.
And without an atmosphere pressing down against the surface of the planet, the pressure at the surface will remain near-vacuum.
However, suppose water does form vapour molecules at the surface of the planet. Why can't the planet hold on to these vapour molecules via its gravity?
How does a planet hold on to the nitrogen gases, the methane and CO2 (that results in becoming an atmosphere) but not to H2O vapour?
Suppose we ignore solar winds : a planet without a master star, or a planet very far away from its master star?
I don't know - we always talk about vapour, but isn't it just liquid particles in air? An aerosol? I've never heard about "gaseous water" - I guess the gas form simply sublimates quickly in air?
Now, wiki tells that water vapour indeed can mean the gaseous form. It invisible, so don't expect clouds.
I don't know - we always talk about vapour, but isn't it just liquid particles in air? An aerosol?
I think yes .. but that's because the atmosphere on Earth is made up of something else, not water .. so evaporated water can be aerosol (certainly if it forms crystals) but if there's no atmosphere, then there's no (gaseous) 'solvent' for the water (vapour) to dissolve into - making it not an aerosol (but 'the' atmosphere instead?)
Especially talking about water - which is so common and fundamental to human being lives - there are loads of non-scientific words for water and it's phases. These work OK on the everyday level but on the science of it, the word used, really matters.
Sublimation for instance is I think quite specific, the phase change from solid to gas ..
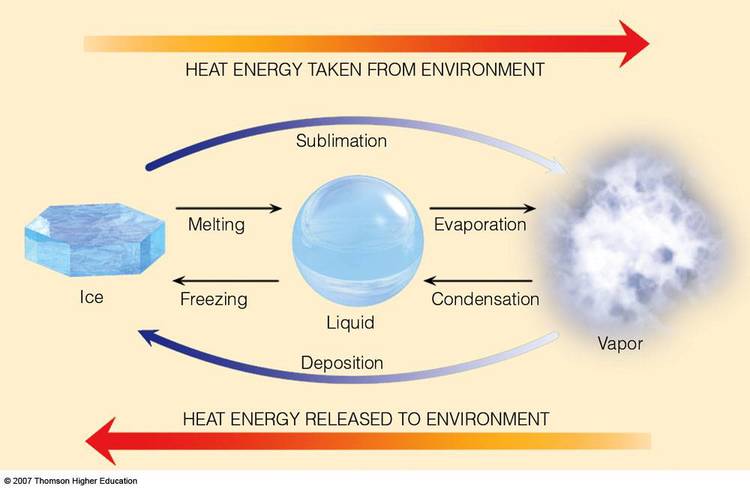
Last edited:
I think yes .. but that's because the atmosphere on Earth is made up of something else, not water .. so evaporated water can be aerosol (certainly if it forms crystals) but if there's no atmosphere, then there's no (gaseous) 'solvent' for the water (vapour) to dissolve into - making it not an aerosol (but 'the' atmosphere instead?)
Especially talking about water - which is so common and fundamental to human being lives - there are loads of non-scientific words for water and it's phases. These work OK on the everyday level but on the science of it, the word used, really matters.
Sublimation for instance is I think quite specific, the phase change from solid to gas ..
Yes, I meant the condensation.
I think yes .. but that's because the atmosphere on Earth is made up of something else, not water .. so evaporated water can be aerosol (certainly if it forms crystals) but if there's no atmosphere, then there's no (gaseous) 'solvent' for the water (vapour) to dissolve into - making it not an aerosol (but 'the' atmosphere instead?)
Especially talking about water - which is so common and fundamental to human being lives - there are loads of non-scientific words for water and it's phases. These work OK on the everyday level but on the science of it, the word used, really matters.
Sublimation for instance is I think quite specific, the phase change from solid to gas ..
So lets say a planet with an icy surface, far away from its sun (to make solar winds negligible) - could have an atmosphere that is made up of water molecules but not dissolved in air because there is no other gas present. Right?
You need a high temperature to evaporate water or even allow for liquid phase. AFAIK the lowest temperature you can get liquid water at would be around 250K or so at quite ridiculous pressure, and you wouldn't get proper vapour once you get there, just a supercritical something. It's not easy to get all threeSo lets say a planet with an icy surface, far away from its sun (to make solar winds negligible) - could have an atmosphere that is made up of water molecules but not dissolved in air because there is no other gas present. Right?
Last edited:
Distance from sun makes no real difference.So lets say a planet with an icy surface, far away from its sun (to make solar winds negligible) - could have an atmosphere that is made up of water molecules but not dissolved in air because there is no other gas present. Right?
While gravity attracts atoms it's the magnetic field that stops them being "blown away" by the solar wind...and for this the planet/moon needs a spinning iron rich core (or metallic hydrogen for large gas giants)
The magnetic field deflects the solar winds to the poles of the planet...we see this visually as the Northern Lights (Aurora Borealis)
With no magnetic field a planet as minimal or no atmosphere since it has no protection from the solar winds.
This is the issue with Mars...it's loosing its magnetic field and over many, many, many years it's slowly loosing it's atmosphere.
Moons, like those around Jupiter, are constantly refreshing their atmosphere with water vapour.
Last edited:
So lets say a planet with an icy surface, far away from its sun (to make solar winds negligible) - could have an atmosphere that is made up of water molecules but not dissolved in air because there is no other gas present. Right?
"Ice" can be carbon dioxide, methane and such, too. It is more likely there'd be a mix of gases, I think. Now about clouds in methane or carbon oxide atmos - I dunno, but that's a thing for dev ideas if they want to go for the atmospheric landings.
I recommend "Fiasko" by Stanislaw Lem - it starts out on Titan (I think) and describes a bizarre moon surface with strange features. If such things can already be modeled satisfyingly in games - who knows.
I recommend "Fiasko" by Stanislaw Lem.
Seconded.
I am curious to the reasons why or why not.
Can anyone volunteer a scientific reasoning?
The answer is 'it depends'.
If you mean liquid water on the surface, then no it won't be possible without an atmosphere - this is because close to a star in a vacuum, liquid water will boil into gaseous form, and either escape into space or be captured as an atmosphere.
Further out from the star, water on the surface would be frozen, so again, no possibility of liquid water on the surface.
However, you didn't specify that the liquid water had to be on the surface - an atmosphere-less planet could have liquid water deep under the surface, because the planetary crust will do the job of providing enough pressure to stop liquid water just boiling off.
Bottom line - it's all about the pressure - the reason we all think water boils at 100c is because that's true under 1 [Earth] atmosphere of pressure - if you took a kettle up Mount Everest, the pressure drop would make the water boil at a much lower temperature (so if you ARE taking a kettle up mt Everest, don't bother taking teabags - it'll taste horrible). If you extrapolate that out to a vacuum, water will vigorously boil away at room temperature - you can find YouTube vids of exactly that (and if you're an adult, you might even remember doing the experiment yourself in high-school physics class! - thank you, Ms Greenway - I knew that lesson would come in handy someday!).
Last edited:
