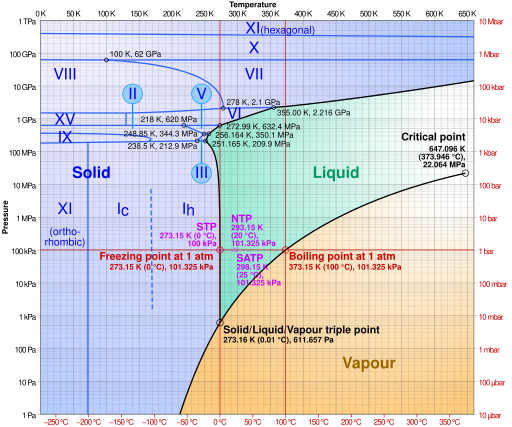
By passing the 20 million amperes of current through a small aluminum chamber, a magnetic field is created that isentropically compresses aluminum plates that sandwich a thin (25 micron) layer of water to pressures ranging from 50,000 to 120,000 atmospheres. For reference, what you experience at sea level is one atmosphere of pressure. What the researchers found was at these incredibly high pressures, water was squeezed into ice—ice VII to be exact, which was subsequently hotter than the boiling point of water at atmospheric pressure. As described by Sandia researcher Daniel Dolan, "Apparently it's virtually impossible to keep water from freezing at pressures beyond 70,000 atmospheres." Maybe that's a bit of an understatement, but it is very important to know for future operation of the Z machine and similar devices. The physical properties of ice—any ice phase—are vastly different from their liquid counter part.